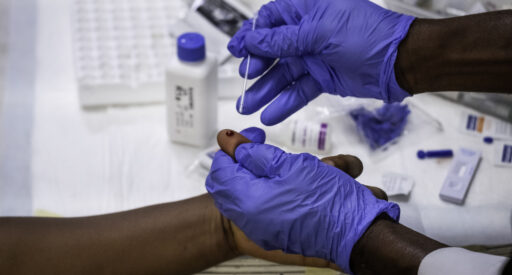
Dual test for HIV and syphilis
Dual tests enable simultaneous testing for HIV and syphilis in pregnant women, helping increase rates of syphilis testing and bringing them closer to those for HIV.
Our volume guarantee has helped SD Biosensor to reduce the price of its dual rapid diagnostic test, enabling more countries to switch to dual testing.
Our
partners
The challenge
Public health
Syphilis is one of several sexually transmitted infections that can be passed from a pregnant woman to her baby during pregnancy and childbirth. Pregnant women with untreated syphilis are 52% more likely to suffer adverse birth outcomes such as stillbirths, low birth weight and premature delivery.
The World Health Organization recommends that all pregnant women are tested for HIV and syphilis during their first antenatal care visit and has set a target to test at least 95% of the pregnant woman who attend. In low- and middle-income countries, despite between 70 and 100% of pregnant women being screened for HIV, only 40 to 60% are screened for syphilis.
Market situation
SD Biosensor developed a dual rapid diagnostic test (RDT) that enables simultaneous diagnosis of HIV and syphilis. The dual test offers an opportunity to integrate syphilis testing with donor-funded HIV testing programmes, which have high testing rates built on well-established guidelines and processes.
By mid-2021, country introductions of the dual test had been slow, primarily due to the price difference between the single HIV test and the dual test. Procurers had indicated that if the price for the test was closer to the price of single HIV tests, it would enable them to significantly increase procurement.
Impact
By the end of 2022:
59,000
pregnant women with syphilis identified
7,900
fewer babies born with congenital syphillis
10,600
stillbirths averted
The product
The dual test is a point-of-care rapid diagnostic test that simultaneously detects antibodies to both Treponemal pallidum (TP, the cause of syphilis infection) and HIV in under 20 minutes from a single finger-prick blood sample.
The dual test means syphilis testing can be integrated into HIV testing programmes in antenatal care settings, closing the testing gap between HIV and syphilis.
The partnership
MedAccess provided a volume guarantee to SD Biosensor for the dual test.
SD Biosensor agreed to reduce the price of its dual test to below $1 for public purchasers in over 100 low- and middle-income countries. SD Biosensor will continue to produce and supply the dual test, increasing availability and access to the product in low-and-middle-income countries.
CHAI helped to facilitate the volume guarantee agreement and continues to support country adoption of the dual test.
Impact
By the end of 2022:
$883,000
direct savings for procurers
<$1
first HIV/syphilis dual test to be offered for under $1
21%
reduction in the average market price of dual tests
Impact projections
Our guarantee enabled SD Biosensor to offer the lowest price among all WHO pre-qualified HIV-syphilis dual test suppliers, encouraging more competitive pricing across the market. SD Biosensor’s market entry made the company third supplier of dual tests in the market, which also contributes to increased supply security.
Bringing the price of the dual test closer to that of single HIV rapid diagnostic tests makes replacing single tests with the dual test a more attractive proposition. By building on existing programmes and systems, we project an additional 4.4 million women will be tested for syphilis who otherwise might have just been tested for HIV.
We estimate that the partnership will contribute to:
- at least 285,000 pregnant women with syphilis being identified,
- at least 245,000 women receiving treatment,
- at least 51,500 stillbirths and miscarriages being avoided
- at least 14,000 fewer babies born with congenital syphilis, and
- a 21% reduction in the average market price of dual rapid diagnostic tests and direct savings of over $3.1 million over the volume guarantee period.
How we calculate the impact of this agreement
Lives changed
Estimations for additional women receiving access to syphilis testing are based on 2020 antenatal testing coverage for syphilis in African countries. Downstream patient outcomes were estimated based on 2020 seroprevalence and treatment rates in African countries, and a meta-analysis of risk for adverse outcomes of pregnancy by Gomez et. al.
Money saved
Impact is based on comparing the negotiated price to the expected weighted average market price of HIV-syphilis dual tests, including expected distributor mark-up where relevant.
Markets shaped
We work with partners, including donors, procurers and ministries of health, to track changes in health markets where our investments are supporting access to products. We monitor for changes to policy, procurement practices and supplier movement, all of which affect markets and contribute to the long-term sustainability of impact.
Sustainable Development Goals (SDGs)

SDG 17
SDG 3
3.2
By 2030, end preventable deaths of newborns and children under 5 years of age, with all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births
3.3
By 2030, end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases and combat hepatitis, water-borne diseases and other communicable diseases
3.7
By 2030, ensure universal access to sexual and reproductive health-care services, including for family planning, information and education, and the integration of reproductive health into national strategies and programmes
3.8
Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all
3.b
Support the research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines, in accordance with the Doha Declaration on the TRIPS Agreement and Public Health, which affirms the right of developing countries to use to the full the provisions in the Agreement on Trade-Related Aspects of Intellectual Property Rights regarding flexibilities to protect public health, and, in particular, provide access to medicines for all
SDG 5
5.6
Ensure universal access to sexual and reproductive health and reproductive rights as agreed in accordance with the Programme of Action of the International Conference on Population and Development and the Beijing Platform for Action and the outcome documents of their review conferences
SDG 10
10a
Implement the principle of special and differential treatment for developing countries, in particular least developed countries, in accordance with World Trade Organization agreements
SDG 17
17.6
Enhance North-South, South-South and triangular regional and international cooperation on and access to science, technology and innovation and enhance knowledge sharing on mutually agreed terms, including through improved coordination among existing mechanisms, in particular at the United Nations level, and through a global technology facilitation mechanism
17.7
Promote the development, transfer, dissemination and diffusion of environmentally sound technologies to developing countries on favourable terms, including on concessional and preferential terms, as mutually agreed
17.10
Promote a universal, rules-based, open, non-discriminatory and equitable multilateral trading system under the World Trade Organization, including through the conclusion of negotiations under its Doha Development Agenda
17.17
Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships
Discover more about this partnership
Key contacts
If you would like more information about this agreement, please reach out to our key contacts.