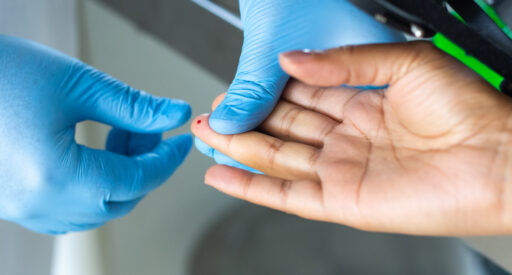
G6PD testing
Testing for G6PD enzyme deficiency is essential for healthcare workers to provide the most appropriate treatment for P. vivax malaria.
Our volume guarantee is helping to ensure the availability of G6PD testing in countries with high P. vivax malaria burden.
Our
partners
The challenge
Public health
The P. vivax malaria parasite caused an estimated 6.9 million cases of malaria in 2022. Complete treatment requires elimination of the parasite from the blood as well as the liver. This treatment is known a ‘radical cure’.
However, doctors are wary of prescribing the most effective treatments because the treatments can cause severe anaemia in patients who are deficient in the enzyme glucose-6 phosphate dehydrogenase (G6PD). This affects 400 million people worldwide.
The World Health Organization (WHO) recommends conducting a G6PD deficiency test prior to treatment of P. vivax malaria patients, to guide treatment decisions. But many countries have not adopted this recommendation meaning point of care G6PD tests are not widely available.
In places with no access to G6PD testing, patients are usually prescribed longer courses of primaquine with lower dosing. However, patients often stop taking the pills when they start to feel better and this can lead to reinfection because the parasite persists in their liver.
Market situation
G6PD testing is required for countries to be able to roll-out tafenoquine, a new, more effective treatment for P. vivax malaria.
But widespread adoption of G6PD testing is limited due to inconsistent supply, low affordability, and the absence of cost-effectiveness, operational, and feasibility studies. In turn, commercial sustainability of the product is threatened by the relatively small market size, uncertain demand, and complexity of production and commercialisation of testing equipment. This has caused some suppliers to exit the market.
SD Biosensor’s STANDARD G6PD Test is currently the only quantitative point-of-care G6PD test on the market. Demand visibility was critical to sustain production levels and support the commercial feasibility of the product, while high-burden priority countries finalise studies, and roll-out G6PD testing in accordance with WHO recommendations.
Impact to date
By the end of 2024:
17,900
additional people on any treatment
5,700
malaria relapses averted
$1.9m
savings for procurers
The product
The G6PD point-of-care test allows doctors to test patients for G6PD deficiency and receive results within two minutes.
The device requires taking a pin prick of blood, which is tested in the analyzer. This looks for quantitative G6PD enzyme activity.
The analyzer is battery-operated and fits in the palm of one hand, so it can be easily stored and moved.
The partnership
MedAccess provided a volume guarantee to SD Biosensor.
SD Biosensor will continue to produce and supply its G6PD testing devices and test strips globally, and offer both products at a reduced price in low-and-middle-income countries.
PATH will continue to work with countries with high P. vivax malaria incidence to increase use of G6PD testing and uptake of ‘radical cure’ treatment.
The new ceiling price is available in certain eligible countries.
Impact projections
Our guarantee will help to ensure the G6PD market remains active ahead of expected market entry by companies with new devices. A healthy market with multiple suppliers will ensure more stable supply of G6PD testing in the future and lead to more competitive pricing.
A more stable G6PD testing market will also support increased prescription of effective treatment, contributing to reductions in P. vivax malaria reinfections and supporting progress towards elimination.
MedAccess projects that sustained access to G6PD testing will allow more patients to receive superior treatment and contribute to malaria elimination targets. We estimate that the partnership will contribute to:
- 9,600 additional people receiving treatment, whereas without testing they wouldn’t have received any medication
- 288,000 additional people receiving superior treatment, leading to an estimated 35,300 fewer malaria relapses
- $134,000 procurement savings for public procurers, due to an average of 10% product price reduction across testing kits and strips.
How we calculate the impact of this agreement
Lives changed
Estimates for treatment eligibility were derived from projections by Watson et al. Estimates of treatment outcomes were derived from a 2020 meta-analysis by Rodrigo et al.
Money saved
Impact is based on actual price reductions for G6PD tests over the course of the volume guarantee.
Markets shaped
We work with partners, including donors, procurers and ministries of health, to track changes in health markets where our investments are supporting access to products. We monitor for changes to policy, procurement practices and supplier movement, all of which affect markets and contribute to the long-term sustainability of impact.
Sustainable Development Goals (SDGs)
SDG 17
SDG 3
3.3
By 2030, end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases and combat hepatitis, water-borne diseases and other communicable diseases
3.8
Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all
3.b
Support the research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines, in accordance with the Doha Declaration on the TRIPS Agreement and Public Health, which affirms the right of developing countries to use to the full the provisions in the Agreement on Trade-Related Aspects of Intellectual Property Rights regarding flexibilities to protect public health, and, in particular, provide access to medicines for all
SDG 10
10a
Implement the principle of special and differential treatment for developing countries, in particular least developed countries, in accordance with World Trade Organization agreements
SDG 17
17.6
Enhance North-South, South-South and triangular regional and international cooperation on and access to science, technology and innovation and enhance knowledge sharing on mutually agreed terms, including through improved coordination among existing mechanisms, in particular at the United Nations level, and through a global technology facilitation mechanism
17.7
Promote the development, transfer, dissemination and diffusion of environmentally sound technologies to developing countries on favourable terms, including on concessional and preferential terms, as mutually agreed
17.10
Promote a universal, rules-based, open, non-discriminatory and equitable multilateral trading system under the World Trade Organization, including through the conclusion of negotiations under its Doha Development Agenda
17.17
Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships
Discover more about this partnership
Key contacts
If you would like more information about this agreement, please reach out to our key contacts.