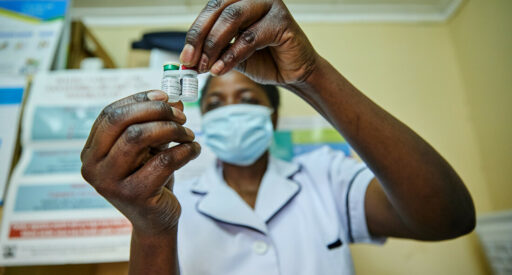
RTS,S malaria vaccine
RTS,S is the world’s first malaria vaccine, designed specifically to tackle malaria in Africa.
Our guarantee was part of an innovative financing arrangement with Gavi and GSK to ensure continued production of RTS,S antigen ahead of key policy and funding decisions.
Our
partners
The challenge
Public health
Malaria is a life-threatening illness caused by parasites transmitted to people bitten by infected female Anopheles mosquitos.
The World Health Organization (WHO) estimates that malaria claimed 619,000 lives in 2021. Africa is disproportionately affected by malaria, accounting for approximately 95% of the cases and deaths worldwide. 80% of malaria deaths in Africa are in children under five.
Although existing interventions have helped to reduce malaria deaths significantly over the past 15 years, a vaccine adds an important complementary tool for malaria control efforts.
Before GSK and partners developed RTS,S, the world had never had a malaria vaccine. The RTS,S vaccine was developed specifically to tackle P. falciparum malaria in Africa.
Market situation
RTS,S received a positive medical opinion from the European Medicines Agency in 2015. At the time of signing this agreement, the vaccine was being piloted in routine immunisation programmes through the World Health Organization’s Malaria Vaccine Implementation Programme (MVIP). Interim data from the pilots was expected in late 2021, which would enable WHO to issue a recommendation for wider use. GSK committed to produce the vaccines required for the MVIP but was unable to promise further production until there was certainty on vaccine roll-out and demand. This required a recommendation for use from WHO and funding commitments from Gavi.
Because the vaccine was to be given as part of routine immunisation for children – rather than campaigns for adults – there was no other viable market for the vaccine. A halt in manufacturing would have resulted in millions of children not having access to the vaccine while production was restarted and scaled up.
The product
The RTS,S/AS01e vaccine was developed by GSK and partners over more than three decades.
It was the first malaria vaccine to successfully complete clinical trials, with an efficacy of 39% against clinical malaria over an 18-month period for children first immunised between the ages of five to 17 months.
Vaccines are an important additional tool in the fight against malaria, especially in areas where access to other tools, such as mosquito nets, may be limited.
The partnership
Gavi agreed to fund GSK’s continued production of the RTS,S antigen for up to three years. If the Gavi Board approved a malaria vaccination programme (following a positive WHO recommendation), GSK would credit the value of the Gavi-funded costs towards procurement of the malaria vaccine doses for the Gavi-supported programme.
If no malaria vaccination programme was approved, MedAccess would replenish Gavi for the majority of costs incurred.
On 6 October 2021, WHO recommended the use of the RTS,S vaccine for prevention of P. falciparum malaria in children living in regions with moderate to high malaria transmission.
On 2 December 2021, the Gavi Board approved a $155.7 million programme to support the introduction, procurement and delivery of the malaria vaccine to Gavi-eligible countries in sub-Saharan Africa.
Impact projections
Our guarantee meant Gavi could provide funding to ensure GSK’s continued production of RTS,S antigen ahead of policy and funding decisions. A halt in production could have meant millions of children in Africa missing out on the vaccine, which will be given as part of routine immunisation. More doses will be available more quickly for country introductions following positive policy and funding decisions from WHO and Gavi.
Projections for this guarantee were based on two-years of guaranteed production, plus catalysed ramp-up made possible by avoiding a factory shutdown. Due to the shorter than expected period of this guarantee, projections will be adjusted based on new data.
We estimate that the partnership will contribute to reaching up to 7.5 million more children than would otherwise have been possible if there was a production delay, contributing to 8.7 million malaria cases averted in children and 36,300 deaths averted.
Immunisation programmes using vaccines supported through the MedAccess-GSK-Gavi agreement are expected to begin in late 2023.
How we calculate the impact of this agreement
All development impact estimates were dependent on the exact timing of WHO’s policy decision and Gavi’s funding decision. We projected impact across various timelines between 2021 and 2028 to ensure we captured the full range of potential impact.
Lives changed
Inputs used to estimate expected additional children vaccinated through the accelerated access to RTS,S include timing of key policy and funding decisions, expected production schedules, and projected demand following a Gavi decision. Deaths and cases averted are estimated using modelling published by Penny et al. in 2016 and assume available vaccine doses will be used to fully vaccinate fewer children rather than partially vaccinate a greater number.
Money saved
Impact is estimated based on the additional costs associated with a stop-and-start production scenario, which was used to calculate an average price per dose for projected doses purchased between 2021 and 2028 across various timelines.
Markets shaped
We work with partners, including donors, procurers and ministries of health, to track changes in health markets where our investments are supporting access to products. We monitor for changes to policy, procurement practices and supplier movement, all of which affect markets and contribute to the long-term sustainability of impact.
Sustainable Development Goals (SDGs)
SDG 17
SDG 3
3.2
By 2030, end preventable deaths of newborns and children under 5 years of age, with all countries aiming to reduce neonatal mortality to at least as low as 12 per 1,000 live births and under-5 mortality to at least as low as 25 per 1,000 live births
3.3
By 2030, end the epidemics of AIDS, tuberculosis, malaria and neglected tropical diseases and combat hepatitis, water-borne diseases and other communicable diseases
3.8
Achieve universal health coverage, including financial risk protection, access to quality essential health-care services and access to safe, effective, quality and affordable essential medicines and vaccines for all
3.b
Support the research and development of vaccines and medicines for the communicable and non-communicable diseases that primarily affect developing countries, provide access to affordable essential medicines and vaccines, in accordance with the Doha Declaration on the TRIPS Agreement and Public Health, which affirms the right of developing countries to use to the full the provisions in the Agreement on Trade-Related Aspects of Intellectual Property Rights regarding flexibilities to protect public health, and, in particular, provide access to medicines for all
SDG 10
10a
Implement the principle of special and differential treatment for developing countries, in particular least developed countries, in accordance with World Trade Organization agreements
SDG 17
17.6
Enhance North-South, South-South and triangular regional and international cooperation on and access to science, technology and innovation and enhance knowledge sharing on mutually agreed terms, including through improved coordination among existing mechanisms, in particular at the United Nations level, and through a global technology facilitation mechanism
17.7
Promote the development, transfer, dissemination and diffusion of environmentally sound technologies to developing countries on favourable terms, including on concessional and preferential terms, as mutually agreed
17.10
Promote a universal, rules-based, open, non-discriminatory and equitable multilateral trading system under the World Trade Organization, including through the conclusion of negotiations under its Doha Development Agenda
17.17
Encourage and promote effective public, public-private and civil society partnerships, building on the experience and resourcing strategies of partnerships
Discover more about this partnership

4 August 2021
New financing agreement boost for malaria vaccine
Key contacts
If you would like more information about this agreement, please reach out to our key contacts.